Remdesivir is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is more than an experimental drug. First developed as treatment for Ebola, it works by confusing the virus as it looks chemically similar to some of the raw materials the virus needs to replicate.It is administered via injection into a vein.
During the 2020 COVID-19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in around 50 countries.
Mechanism of Action
As an adenosine nucleoside triphosphate analog (GS-443902), the active metabolite of remdesivir interferes with the action of viral RNA-dependent RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production. RNA polymerase plays a very important role in the process of RNA replication, and SARS-CoV2 is RNA virus.
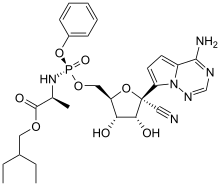
In some viruses such as the respiratory syncytial virus it causes the RNA-dependent RNA polymerases to pause, but its predominant effect (as in Ebola) is to induce an irreversible chain termination. Unlike with many other chain terminators, this is not mediated by preventing addition of the immediately subsequent nucleotide, but is instead delayed, occurring after five additional bases have been added to the growing RNA chain.
For the RNA-Dependent RNA Polymerase of MERS-CoV, SARS-CoV-1, and SARS-CoV-2 arrest of RNA synthesis occurs after incorporation of three additional nucleotides. Hence, remdesivir is classified as a direct-acting antiviral agent that works as a delayed chain terminator.
Compassionate Use
On 20 March 2020, United States President Donald Trump announced that remdesivir was available for “compassionate use” for people with COVID‑19; FDA Commissioner Stephen Hahn confirmed the statement at the same press conference. It was later revealed that Gilead had been providing remdesivir in response to compassionate use requests since 25 January. On 23 March 2020, Gilead voluntarily suspended access for compassionate use (excepting cases of critically ill children and pregnant women), for reasons related to supply, citing the need to continue to provide the agent for testing in clinical trials.

On 1 May 2020, the US Food and Drug Administration granted Gilead emergency use authorization (EUA) for remdesivir to be distributed and used by licensed health care providers to treat adults and children hospitalized with severe COVID‐19.
On 28 August 2020, the FDA broadened the Emergency Use Authorization (EUA) for remdesivir to include all hospitalized patients with suspected or laboratory-confirmed COVID‑19, irrespective of the severity of their disease. The Fact Sheet was updated to reflect the new guidance.
On 1 October 2020, Gilead and HHS announced that HHS was relinquishing control over remdesivir allocation because production of the drug had finally caught up with US domestic demand. AmerisourceBergen will remain the sole distributor of Veklury in the US through the end of 2020.
On 22 October 2020, the FDA approved remdesivir and also revised the EUA to permit the use of remdesivir for treatment of suspected or laboratory confirmed COVID‑19 in hospitalised children weighing 3.5 kilograms (7.7 lb) to less than 40 kilograms (88 lb) or hospitalized children less than twelve years of age weighing at least 3.5 kilograms (7.7 lb).
In November 2020, the FDA issued an EUA for the combination of baricitinib with remdesivir, for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized people two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). The data supporting the EUA for baricitinib combined with remdesivir are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), which was conducted by the National Institute of Allergy and Infectious Diseases (NIAID). The EUA was issued to Eli Lilly and Company.
Mechanism of Action

In this image released by the White House, President Donald Trump works in the Presidential Suite at Walter Reed National Military Medical Center in Bethesda, Maryland Saturday, October 3, after testing positive for the coronavirus.
For the treatment of the President
After the U.S. President Donald Trump was infected with SARS-CoV2, his personal doctor revealed that he had been treated with the Gilead antiviral. Mr Trump also took monoclonal antibody therapy made by the company Regeneron. The antibodies physically stick to the coronavirus so they cannot get inside the body’s cells. It took a total of three days for President Trump to be cured of SARS-CoV2 after being treated with remdesivir at a very high concentration.
For the treatment of Rudy Giuliani
President Donald Trump’s personal lawyer, Rudy Giuliani has revealed in a call to his own radio show that he is being treated for coronavirus with the same drug cocktail his boss received when he was ill with COVID-19. He was admitted to hospital on Sunday after becoming the latest official close to President Trump to be tested positive.

Mr Giuliani, 76, told the show he expects to leave hospital on Wednesday. He has been treated with remdesivir and dexamethasone, he explained.
President Trump tweeted on Sunday that his ally, who has been leading the Trump campaign’s legal challenges to the November election outcome, had been diagnosed with the virus.
“I am doing fine. Pretty much all the symptoms are gone. The minute I took the cocktail I felt 100% better. It works very quickly, wow,” he told his colleagues on his weekly show with 77 WABC radio from the Medstar Georgetown University Hospital in Washington DC.
References:
Remdesivir |WIKIPEDIA https://en.wikipedia.org/wiki/Remdesivir
Trump lawyer Rudy Giuliani receiving same COVID drugs as president |BBC NEWS https://www.bbc.com/news/world-55243581


